Research data showed that children are less likely to become seriously ill. The emergence of Delta variant has been has changed that, destroying the myth that healthy kids can’t get hit hard by the virus.
While many high-income nations now offer Covid vaccines for children 12 and older, a handful of countries have now authorized the shot for younger people.
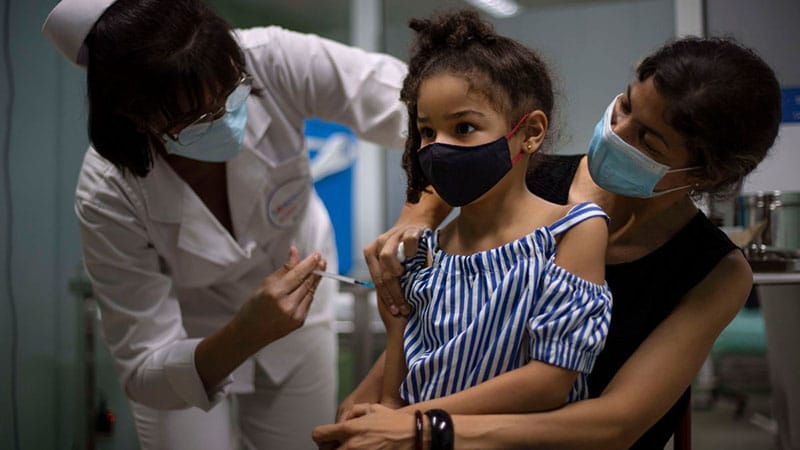
Cuba has became the first country in the world to vaccinate children as young as 2 this September, vaccinating with their homegrown vaccines, which the government claims are safe for younger kids. The island nation initially planned to focus on vaccinating healthcare workers, the elderly and the hardest-hit areas. Then, following a spike in infections among children attributable to Delta, it announced that it would also prioritize young children in a bid to safely reopen classrooms.
Cuba has yet to provide data on its vaccines to outside observers, but has said it will seek World Health Organization (WHO) approval on Thursday.
Chile, China, El Salvador and the United Arab Emirates have also approved vaccines for younger children. In Chile, children aged 6 and older can get the Sinovac shot, while in China, the Sinovac and CoronaVac vaccines are authorized for use in children as young as 3. In El Salvador, children as young as 6 will soon be able to get vaccinated, while in the United Arab Emirates — where Sinopharm is approved for 3-year-olds — the government has made it clear that the vaccination program will be optional.
Meanwhile, American children between 5 and 11 could be eligible for the vaccine sometime this fall, pending approval from the US Food and Drug Administration. Pfizer’s CEO said Tuesday that the company plans to submit data on its vaccine from studies involving that age group by the end of this month.
The United Kingdom has been more cautious than many other European countries in regard to vaccinating younger populations, only recommending the shot for 12-15 year olds on Monday, following advice from its chief medical officers.
In late May, the European Medicines Agency (EMA) approved the use of Pfizer/BioNtech’s vaccine for children aged 12-15, based on a trial that showed that the immune response to the vaccine in that age group was comparable to the immune response seen in people from 16-25. The EMA approved the Moderna vaccine for 12-15 year olds in late July.
France, Denmark, Germany, Ireland, Italy, Spain and Poland are among EU countries that have rolled out their vaccination campaigns for 12-15 year olds, with uptake varying across the bloc.
Switzerland — which is not part of the EU — has been vaccinating the younger age group since June. Sweden will offer the vaccine to 12-15 year-olds later in the fall, Sweden’s Prime Minister Stefan Lofven said Thursday.
Meanwhile, in the UK there are no current plans to vaccinate children under 12, according to Chris Whitty, England’s chief medical officer.